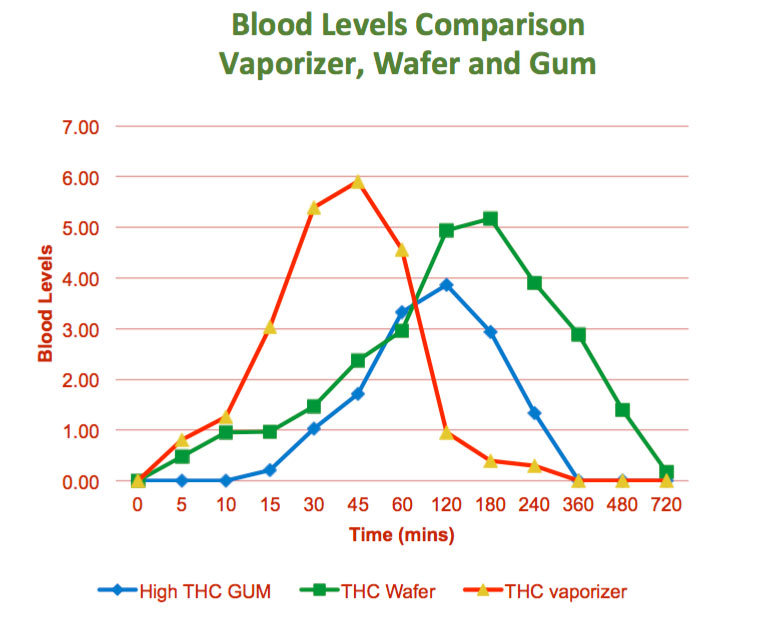
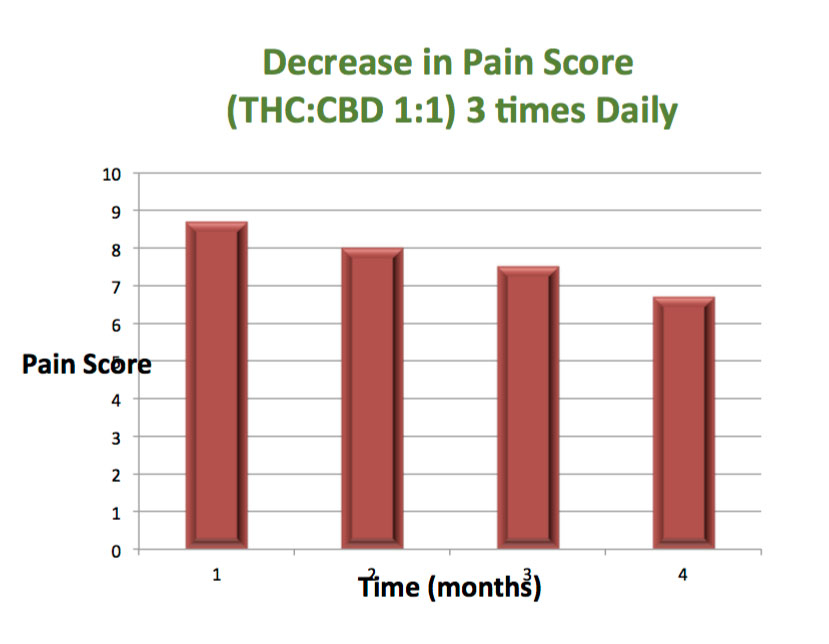
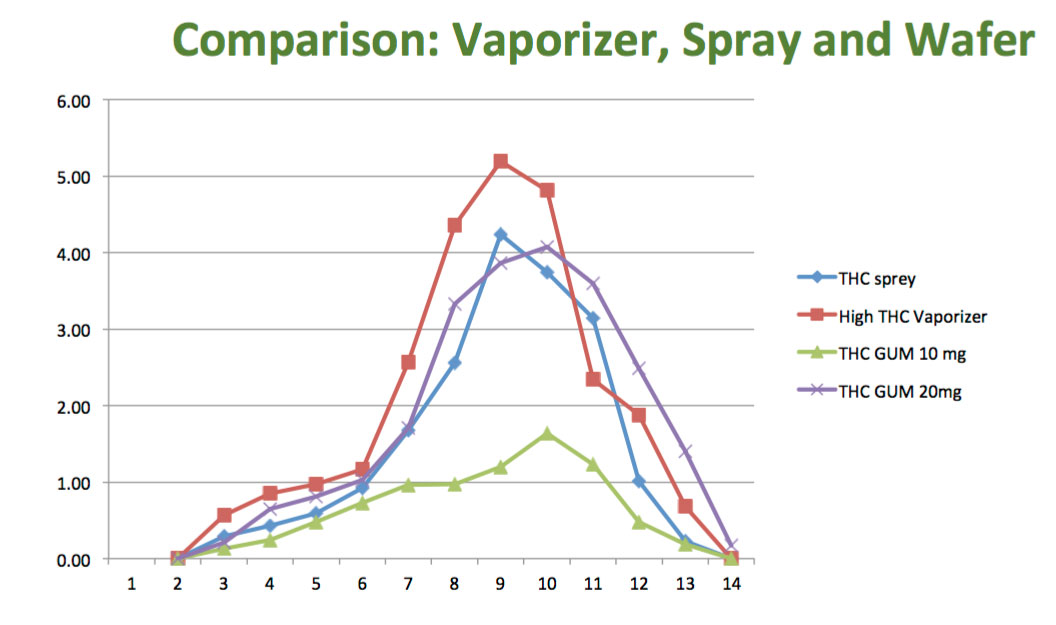
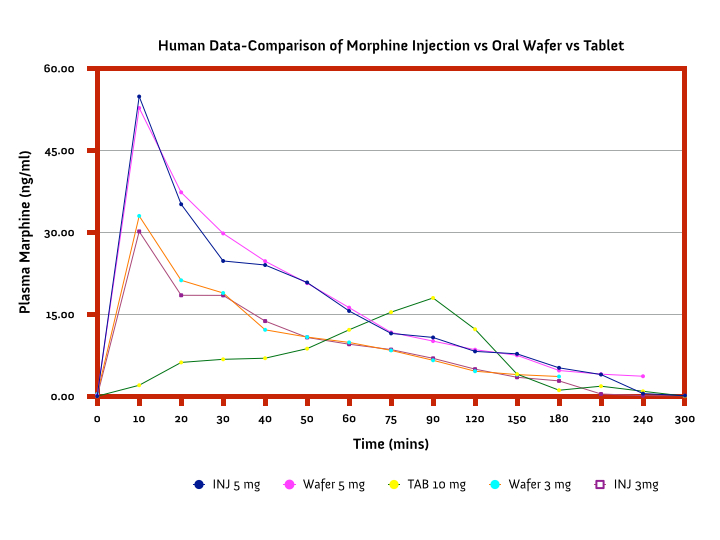
The purpose of this study was to determine in a randomized, crossover manner, the efficacy (effect, or bio-availability) of a Cannabis wafers containing THC/CBD medica,on (pain relieving agents). The secondary objective was to assess the safety, tolerability and the side effects of various doses with the onset of action.
The study was a opened label, randomized, crossover, and dose ranging comparative study of Wafer, Vaporizer and THC wafers administered on different days in 13 healthy subjects (7 men and 6 women).
The wafer was only 1.5-2 cm and approximately 0.05 mm thick. The wafer is made from the FDA approved non-medical ingredients used in several pharmaceutical formulations. The trials were conducted at a university in Spain.